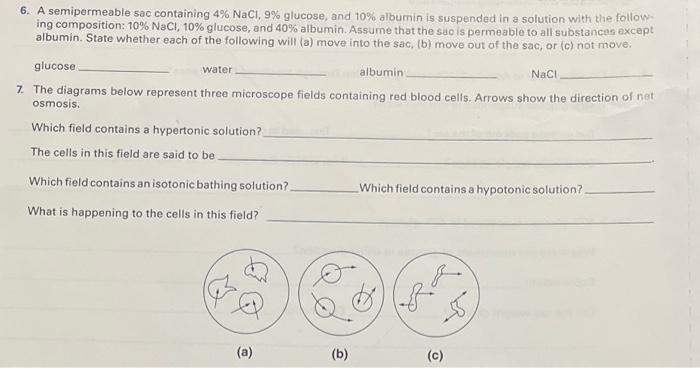
A semipermeable sac containing 4 NaCl is a fascinating scientific concept with wide-ranging implications in various fields. This article delves into the intricacies of this unique structure, exploring its characteristics, functions, and practical applications.
Composed of a selectively permeable membrane, a semipermeable sac allows the passage of certain substances while restricting others. This property makes it a crucial component in biological systems and has led to its use in various technological applications, including dialysis and drug delivery.
Semipermeable Sac

A semipermeable sac is a thin membrane that allows certain molecules or ions to pass through it while blocking others. It acts as a selective barrier, permitting the movement of small molecules like water and ions while restricting the passage of larger molecules or charged particles.
In this article, we will explore the characteristics, functions, and applications of semipermeable sacs, particularly focusing on a semipermeable sac containing 4 NaCl.
Characteristics of the Semipermeable Sac, A semipermeable sac containing 4 nacl
The semipermeable sac in this experiment contains 4 NaCl. This means that the sac contains a solution of sodium chloride (NaCl) with a concentration of 4 moles per liter.
The semipermeable sac is made of a material that allows water molecules to pass through it but does not allow sodium or chloride ions to pass through it. This means that the water molecules can move freely in and out of the sac, but the sodium and chloride ions cannot.
Functions of the Sac
The semipermeable sac plays a crucial role in osmosis, a process by which water molecules move from an area of low solute concentration to an area of high solute concentration.
In the case of the semipermeable sac containing 4 NaCl, water molecules will move from the outside of the sac, where the solute concentration is lower, to the inside of the sac, where the solute concentration is higher.
This movement of water molecules will continue until the solute concentration inside and outside the sac is equal.
Examples of Semipermeable Sacs in Biological Systems
Semipermeable sacs are found in many biological systems, including cells.
The cell membrane is a semipermeable sac that surrounds the cell and controls the movement of molecules into and out of the cell.
The cell membrane allows water molecules, oxygen, and other small molecules to pass through it, but it does not allow larger molecules, such as proteins and DNA, to pass through it.
Applications of Semipermeable Sacs
Semipermeable sacs have a wide range of applications, including dialysis and drug delivery.
In dialysis, semipermeable sacs are used to remove waste products from the blood of patients with kidney failure.
In drug delivery, semipermeable sacs are used to deliver drugs to specific parts of the body.
FAQ Resource: A Semipermeable Sac Containing 4 Nacl
What is the significance of the 4 NaCl in the sac?
The presence of 4 NaCl in the sac creates a concentration gradient, which drives the movement of water molecules across the semipermeable membrane through osmosis.
How does a semipermeable sac contribute to cellular processes?
Semipermeable sacs are essential for maintaining the proper functioning of cells by regulating the exchange of nutrients and waste products, as well as maintaining osmotic balance.
What are the potential applications of semipermeable sacs in drug delivery?
Semipermeable sacs can be used to encapsulate and deliver drugs to specific target sites within the body, improving drug efficacy and reducing side effects.